Catalyst for Hydration of Alkene
Oil fractions freed of these noxious elements are cracked over solid acid catalysts entry 9. Presence of acid as catalyst to form alcohols.

Organic Chemistry Why Does Hydration Of Alkene Takes Place In Presence Of An Acid Such As H3po4 Or H2so4 Chemistry Stack Exchange
The product has the same regiochemistry as an alcohol formed by direct hydration of the same alkene.

. Alkene is often used as synonym of olefin that is any hydrocarbon containing one or more double bonds. When 2-butyne is treate with H2Lindlars catalyst compound X is produced as the major product and when treated with N. Step 1Protonation of alkene to form carbocation by electrophilic attack of H3O.
Catalysts for polymerization reactions Ziegler-Natta catalysts. Functionalized allyl alcohol in the case of aldehyde as the electrophile. Also called α-olefins terminal alkenes are more useful.
Mechanism The mechanism of the reaction involves the following three steps. The alkene affords the compound B CH3 - CH CH - CH3 buildrel O_3 ov. Citation neededHowever the International Union of.
Ch08 Reacns of Alkenes landscape Page 12. The BaylisHillman reaction is a carbon-carbon bond forming reaction between the α-position of an activated alkene and a carbon electrophile such as an aldehyde. The crucial component of modern cracking catalysts is a zeolite synthetic type Y faujasite which contains protonic sites of sufficient acidity to initiate catalysis by converting minority alkene molecules to adsorbed carbocations Chen et al.
JEE Main 2018 Online 15th April Evening Slot. In organic chemistry an alkene is a hydrocarbon containing a carboncarbon double bond. Employing a nucleophilic catalyst such as a tertiary amine and phosphine this reaction provides a densely functionalized product eg.
Both hydroboration-oxidation and acid-catalyzed hydration may result in rearrangement products. Acid catalyzed hydration of alkenes except ethene leads to the. The mechanism of dehydration may vary from alcohol to alcohol even when the same catalyst is being used.
Hydroboration-oxidation results in syn addition of H and OH whereas acid-catalyzed hydration results in anti addition. In case of unsymmetrical alkenes the addition reaction takes place in accordance with Markovnikovs rule Unit 13 Class XI. When the catalyst is in a different physical state to the other reactants it is called Heterogeneous catalysis Eg.
A solid catalyst with a liquid and a gas. 224-trimethylpentane is often added to petrol to enhance its anti-knock properties now that methyl t-butyl ether MTBE is being phased out. Hydroboration oxidation of an unsymmetrical alkene results in the less substituted alcohol product.
The dehydration of alcohol series done by Thomke over BPO₄ Ca₃PO₄₂ and Sm₂O₃ determined the mechanism by two precise criteria uptake of deuterium from deuterated catalysts into produced olefin and un-reacted alcohol. Two general types of monoalkenes are distinguished. The alkene is then hydrogenated using nickel as the catalyst to 224-trimethylpentane isooctane.
H2O H.
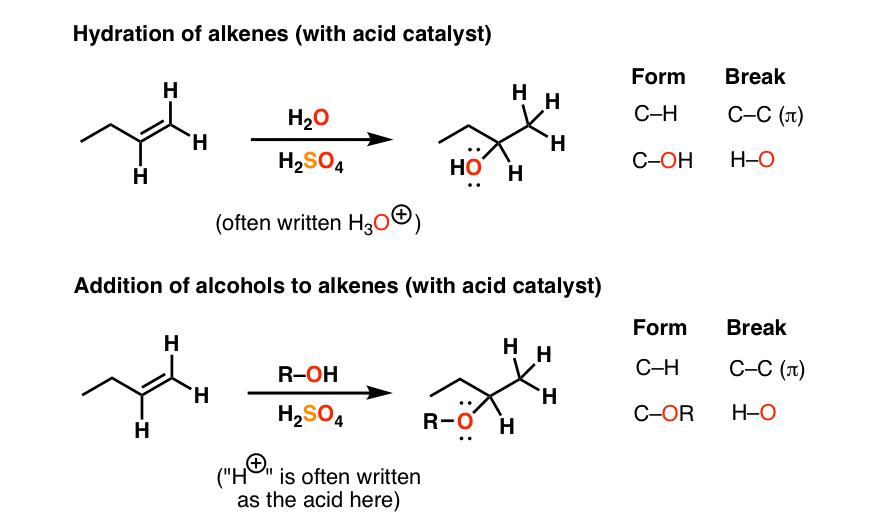
Acid Catalyzed Hydration Of Alkenes Follows A Key Mechanistic Pattern

Hydration Of Alkenes Oxymercuration Hydroboration Chad S Prep
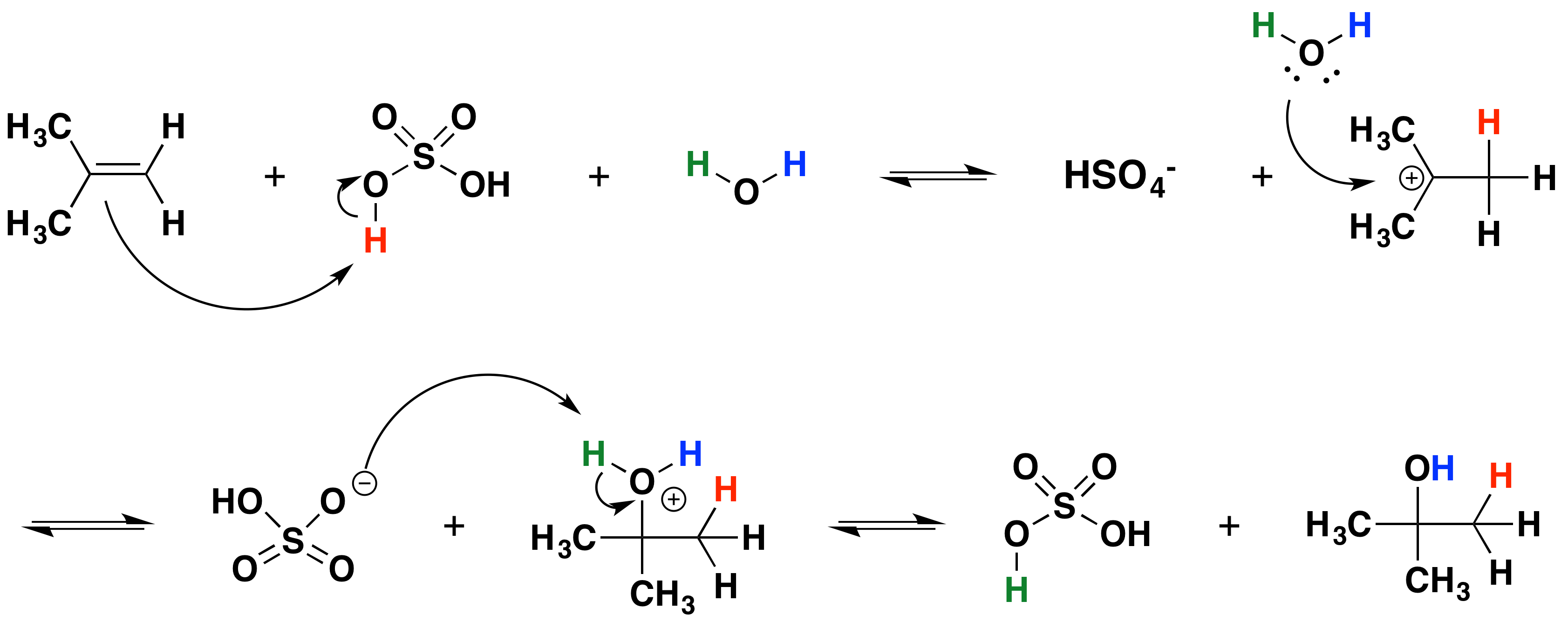
9 5 Electrophilic Hydration To Make Alcohols Chemistry Libretexts
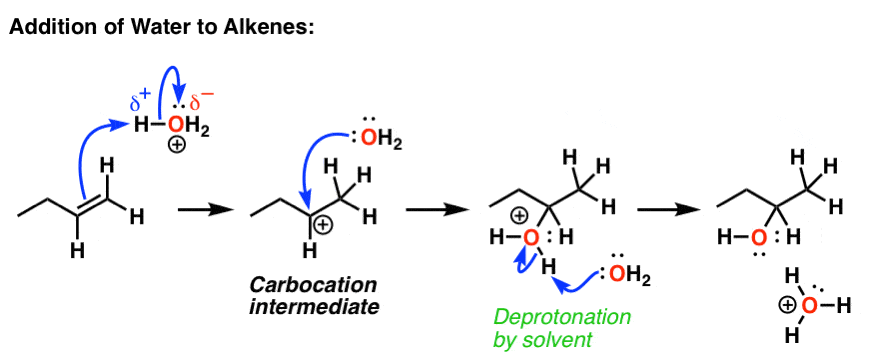
Acid Catalyzed Hydration Of Alkenes Follows A Key Mechanistic Pattern
0 Response to "Catalyst for Hydration of Alkene"
Post a Comment